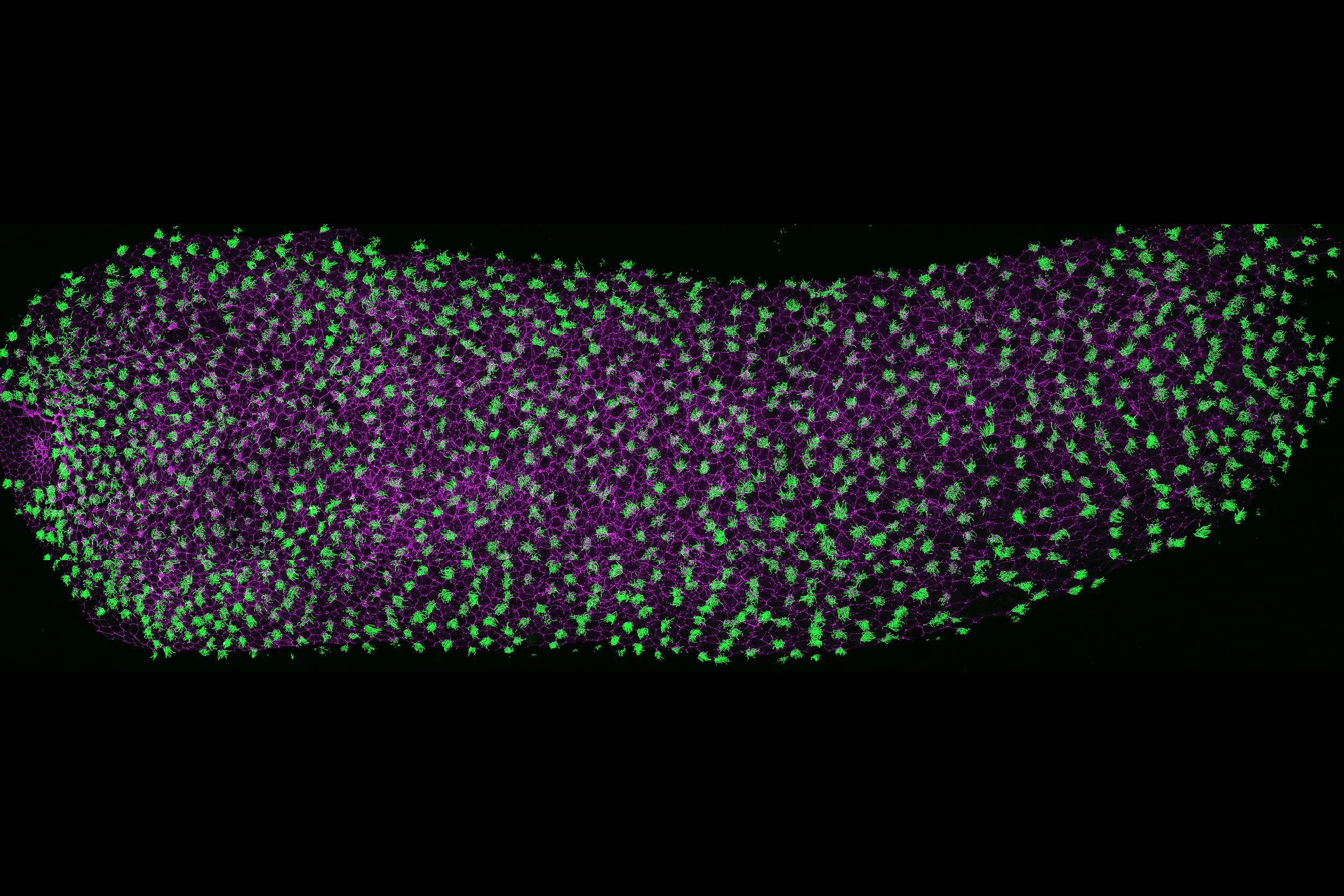
UNLOCKING THE GENETIC CODE TO THE HEART, LUNG AND BRAIN DEFECTS.
Every four minutes, a child is born with a birth defect, often with an unknown genetic cause, leaving patients, parents, and clinicians to navigate uncertainty regarding risk, treatment, and recurrence. OUR MISSION is to address this gap by combining human genetics with in vivo and in vitro disease modeling to uncover the mechanisms of congenital anomalies of the heart, lungs, and brain, paving the way for accurate diagnosis and new therapeutic strategies.
RESEARCH
VISION
To be at the forefront of the cutting-edge field of FUNCTIONAL GENOMICS, enabling scientific curiosity, excellence in research, and groundbreaking discoveries to improve the clinical outcomes for babies born with heart, lung and brain defects.
WHAT IS FUNCTIONAL GENOMICS
Functional genomics is the science of uncovering how genes actually work in living systems, not just which genes are present, but how they control cellular behaviors and shape development. By integrating genome engineering, multi-omics, imaging, and computational analysis, we uncover how gene networks build the heart, lungs, and brain, and how their disruption leads to birth defects and disease- placing functional genomics at the very center of precision medicine as the bridge between genetic information and truly personalized diagnosis, prognosis, and therapy.
Functional genomics is at the leading edge of biomedical science because it combines big data with with deep mechanistic understanding. As a trainee, you will:
Learn state-of-the-art methods like CRISPR screening, single-cell omics, and precision disease modeling.
Gain skills that span cell and developmental biology, human genetics, and systems biology.
Work on high-impact problems that bridge bench science with real-world medicine.
Be part of a collaborative and interdisciplinary team working to redefine how we study, diagnose, and ultimately treat complex human disease.
PROJECTS
Microtubules play a central role in nearly every cellular process, from cell division and structural organization to intracellular transport and signaling. Our lab focuses on two specialized, microtubule-based structures: centrioles and cilia. Centrioles are essential for centrosome assembly, organizing cytoskeleton and mitotic spindles, ensuring accurate cell division and polarity. Cilia, on the other hand, are dynamic, antenna-like projections that are vital for both cellular signaling and motility across tissues. Disruption of either centrioles or cilia can have broad consequences, leading to a range of syndromic disorders, including congenital heart defects (CHD), primary ciliary dyskinesia (PCD), and neurodevelopmental diseases such as microcephaly and autism. By studying these organelles and the gene regulatory networks essential for their formation and function, we aim to uncover the molecular logic that connects cell biology to human health and disease.
Cardio-Pulmonary Genomics
Personalized medicine promises to transform care for congenital heart and lung diseases, but our ability to translate genotype data into actionable insights remains a central obstacle. This gap is especially challenging for disorders involving cilia and centrosome function, processes essential for left-right patterning, heart development, and airway and lung function.
Our laboratory uses integrated functional genomics as our central strategy to tackle this challenge. We employ CRISPR-based genome engineering, high-content multi-omics, and quantitative phenotyping across frogs, mouse models, and human cell systems to examine how specific gene regulatory changes influence organ development. Instead of relying solely on genetic association or prediction, our functional genomics platforms allow us to systematically perturb genes, measure real phenotypic consequences, and map the gene regulatory networks critical for cardiopulmonary development.
By combining these approaches, we move beyond simply listing mutations to uncover how ciliary and centrosomal defects lead to congenital heart defects (CHD), primary ciliary dyskinesia (PCD), and related syndromes. This not only accelerates gene discovery and disease modeling but also offers a strong training environment for preparing the next generation of scientists (you) to lead at the intersection of functional genomics, developmental biology, and precision medicine.
Neurogenetics
Proper brain development depends on the precise regulation of neural progenitor behavior, polarity, and signaling, roles governed by centrioles, centrosomes, and cilia. Indeed, defects in these organelles are linked to neurodevelopmental disorders such as microcephaly and autism. Yet, with hundreds of genes contributing to centriole and cilia function, deciphering the connection between genotype and clinical phenotype remains a major barrier in neuroscience.
Our lab meets this challenge by integrating functional genomics into neurobiology research. We combine CRISPR-based genome editing, single-cell and spatial multi-omics, and advanced quantitative imaging in frogs, mouse models, and human cell systems to systematically study how gene and regulatory network disruptions affect neural progenitor behavior, brain size, and tissue organization.
Moving beyond simple genetic associations, our integrated platforms enable us to explore the cellular and molecular mechanisms behind neurodevelopmental disorders. This approach not only clarifies disease etiology at an unprecedented level but also facilitates rigorous benchmarking of computational predictions against experimentally validated biology. Trainees in our lab thrive in a cross-disciplinary environment where functional genomics drives both discovery and translation, preparing them to lead the next wave of precision neuroscience.
RESOURCES
MICROSCOPY
NIKON AX-R confocal: This new state-of-the-art microscope is equipped with 8K Galvo and 2K resonant scanners, 4 solid-state laser lines (405, 488, 561, and 640), and 4 PMT detectors with 2 GaAsP detectors. The software capabilities include advanced 2D tracking of the cells or organelles, 3D measurement analysis, deconvolution, and tile scanning.
The LEICA SP8 confocal microscope: The microscope is built off a DMi8 inverted research microscope and comes equipped with a white light laser scan head that is tunable within the range of 470-670nm with up to eight laser lines; a 405nm laser; a filter-free spectral detector for up to five individually regulatable channels.
NIKON SMZ1270 Stereomicroscope: The lab has two sets of stereomicroscopes equipped with a camera and computer for high-speed imaging.
TOOLS
High-Throughput CRISPR-Cas9 and morpholino based screening
Imaging: Confocal and Super-resolution microscopy, live imaging, Scanning Electron Microscopy (SEM), Transmission electron microscopy (TEM), and Electron Tomography (ET)
“Omics”: Single cell/nuclei sequencing and proteomics
Computational biology: Mathematical modeling and machine learning
Mechanobiology: Biomechanical manipulations of cells and skin organoids.
MODELS
MEET THE TEAM
-

Saurabh Kulkarni
Assistant Professor
-

Dana Urbatsch
Lab manager
-

Angelo Arrigo
Graduate Student
-

Savanna Hinson
Graduate Student
-

Venkatraman Rao
Senior Postdoctoral Associate
-

Vani Narayan
Postdoctoral Associate
-

Victoria Hua
Undergraduate Researcher
-
Anburaj Jeyraj
Research Associate
AFFILIATIONS
DEVELOPMENTAL GENOMICS CENTER
The Developmental Genomics Center at UVA will bridge developmental biologists with genomic and clinical translational scientists across grounds and with nearby Inova Health System and the NIH NICHD. The Center aims to integrate genomic technologies and next-generation sequencing datasets from human and animal model systems to address cutting-edge research questions in cell and developmental biology.
CENTER FOR MEMBRANE AND CELL PHYSIOLOGY
UVA’s Center for Membrane and Cell Physiology strives to understand fundamental biological processes at the highest possible spatial and temporal resolution. Our ultimate goal is to use high-end imaging, structural, biophysical, and biological and chemical probe technologies to make impactful discoveries on understanding the causes, development, and cures of diseases ranging from cardiovascular to cancer to neurological and infectious diseases.
CHILD HEALTH RESEARCH CENTER
The center's mission is to support scientists engaged in basic and clinical research to discover innovative therapies for childhood diseases. We bridge the gap between the laboratory and the bedside with cutting-edge research that improves the lives of children.
GRADUATE PROGRAMS AND TRAINING GRANTS
The Kulkarni lab is affiliated with postdoctoral and graduate T-32 training grants supported by the National Institutes of Health at the University of Virginia. Post-doctoral and Ph.D. applicants who are interested in the Kulkarni lab may be good candidates for applying for financial support from these training grants.

CONTACT
Kulkarni lab
Department of Cell Biology
Department of Biology,
University of Virginia
Charlottesville, VA 22908
Email: sk4xq@virginia.edu
Phone No: +14342976833

